Multiple Solutions
Multiple Solutions.
Fresenius Kabi is proud to offer products designed to support healthcare professionals in a variety of clinical settings. Through our extensive research and development we have developed a comprehensive portfolio of products spanning blood collection, transfusion medicines, cell processing and much, much more.
Visit us at ISBT 2024 – Booth #D10
Attend and Engage: Industry Symposium – 24, June
Experience and Explore Technology Advancements:
- Alternatives to DEHP in Blood Bags (not available in the US)
- New – CompoGuard Advance (not available in the US)
- AmiCORE Apheresis now with the DXT Data Management System
![]()
Explore Alternatives to DEHP in Blood Bags
We’re Bringing the Next Piece of the Puzzle
Thanks to a smart combination of carefully selected blood bag materials and storage solution, we now offer an RCC in-line system not made with DEHP.*
This is the next puzzle piece in providing alternatives to DEHP in our blood bag portfolio. It allows facilities to continue their current blood processing procedures including storage of red blood cells up to 42 days in PAGGS-M. Initial in vitro and in vivo studies have shown promising results in the quality of blood components based on EDQM guidelines.**
Configurations not made with DEHP include:
- RCC T&B in-line systems
- RCC/PLS double filter in-line systems
- Whole Blood in-line systems
CompoStop Leukocyte Depletion Filter Systems
- For pooled buffy coat
- For pediatric RCC/platelets and for platelet pooling
*Containing less than 0.1% of DEHP by weight of the plasticized material in accordance with Regulation (EC) 1907/2006 (REACH). These products are not available in the United States.
**Vermeulen C et al. Vox Sanguinis. 2022;117:1163–1170
Alternatives to DEHP in Blood Bags: View or Download
Experience Advancements Designed with You in Mind
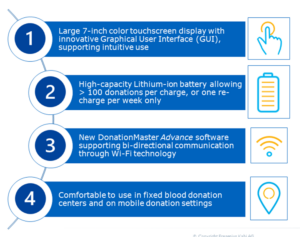
CompoGuard Advance
Whole blood donation mixing & weighing device combined with Wi-Fi Data Management
(Not available in the United States)
CompoGuard Advance offers automated whole blood donation combined with the bi-directional Wi-Fi data management software DonationMaster Advance.
User interface designed for intuitive operations
- Large 7-inch color touchscreen guides users through the donation process
- New, graphical user interface with visible program steps supporting intuitive operations
Equipped for mobile donation
- A robust transport case made of high-indurance plastic designed for transporting the device to a mobile donation setting
- The transport case also functions as a table during donation
- The transport case has space for all necessary equipment (e.g. barcode scanner, power adapter, tube sealer and battery
CompoGuard Advance: View or Download:
Top Features
AmiCORE Apheresis System with DXT Data Management
Designed for Donor and Operator
The AmiCORE Apheresis System is an automated blood cell separator indicated for the collection of blood components with options to collect:
- Plateletpheresis (single, double, or triple units), Leukocytes reduced
- Concurrent plasma
- Platelets with automated addition of Platelet Additive Solution
Simple & Smart
- Configurable disinfection timer allows three phase disinfection timer to scrub and dry venipuncture site consistently and minimize the concern of bacterial contamination
- Sample tube scanning streamlines the donation process throughout
- Saline Prime and Infusion replaces fluid during donation
- Numerical flow rate control and real-time pressure monitoring help protect the donor’s vein and maintain comfort
AmiCORE Apheresis System: View or Download
DXT Data Management System
The DXT Data Management System offers tools essential to oversee daily operations.
Data-Driven Insights
Real-time access to dashboards and reports to review, analyze and make adjustments on the spot, or track data over time to identify opportunities for improved performance.
Bi-directional Interface
DXT’s bi-directional interface helps reduce data entry and documentation errors which may cause avoidable product discards. The bi-directional interface is designed to communicate between your existing computer system and Fresenius Kabi devices and laboratory instruments.
- AmiCORE Apheresis System
- Amicus Separator
- Aurora Plasmapheresis System
- CompoMat G5 Plus
- Lovo Cell Processing (for laboratory use only)
DXT Data Management: View or Download
Engage
Industry Symposium
Latest Trends in Blood Banking – Storage, Transportation and Traceability
ISBT Congress, 24, June from 12:15 to 13:15 pm, room 111 – level P1
Topics
Session: Cold-Stored Platelets – An Outlook from the American Red Cross: A Blast from the Past
Speaker: Dr. Bethany L Brown, American Red Cross
Session: Validation and Planning of Blood Bag Systems not made with DEHP in a Hospital Blood Bank
Speaker: Dr. Francesco Bennardello, Dir. Immunohematology and Transfusion Medicine Service
Session: Non-DEHP Database Organization and Management by the European Blood Alliance
Speaker: Ryan Evans, Scottish National Blood Transfusion Service
Session: 5 Years of Experience with RFID Labels at Brescia Blood Bank, Italy
Speaker: Dr. Susanna Bresciani, Manager of the SIMT Spedali Civili di Brescia, Brescia
NOTE
Not all products listed or shown are approved for sale, or available in all countries. Contact your local Fresenius Kabi Representative for additional information and product availability.
Refer to the product operator’s manual, instructions for use, and other associated labeling and information for a complete list of warnings, cautions, contraindications, and other pertinent information associated with the use of the products listed on this site.
DISCLAIMER
The Amicus System for ECP has obtained CE mark approval for the indication of CTCL in the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma (CTCL) that is unresponsive to other forms of treatment.
The Amicus ECP System is CE marked in compliance with the applicable requirements of the Medical Device Regulation (EU) 2017/745 and includes:
- Amicus Separator
- Phelix Photoactivation Device (Not available in the United States)
- Amicus ECP Apheresis Kits
ECP is not cleared for market in the US.